Optinose Awaits FDA Verdict on Expansion for Chronic Rhinosinusitis Treatment
Yardley-based Optinose, known for its drug-device product Xhance, is at a pivotal moment as it awaits a decision from the Food and Drug Administration, writes John George for the Philadelphia Business Journal.
Seven years following its initial approval for treating nasal polyps, the FDA is now reviewing a supplemental new drug application that could significantly broaden Xhance’s market.
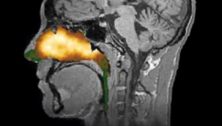
This expansion would allow its use for chronic rhinosinusitis, a condition affecting nearly 30 million Americans, with no FDA-approved treatments available.
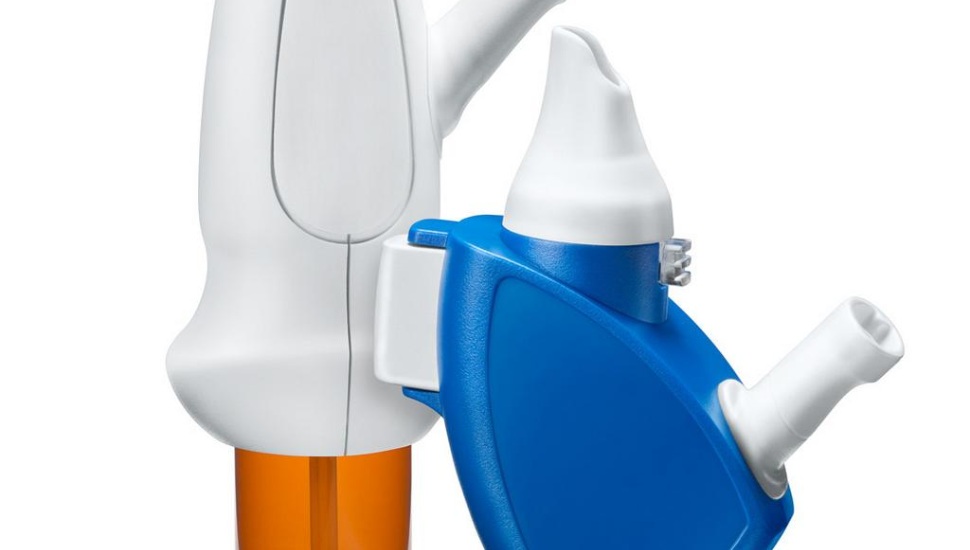
Xhance combines an anti-inflammatory medication with a proprietary exhalation delivery system, aiming to effectively target deep nasal passages.
Despite a slight decline in sales in 2023, dropping to $71 million from the previous year’s $76.3 million, the potential approval for treating chronic rhinosinusitis could transform Optinose’s business landscape.
Chronic rhinosinusitis, marked by prolonged inflammation of the nasal and sinus cavities, leads to symptoms like congestion, facial pain, and diminished senses of smell and taste. Currently, only a fraction of those suffering seek treatment.
Read more about Yardley-based Optinose and its efforts to expand its treatment visit the Philadelphia Business Journal.
Optinose CEO: Building Awareness | Mad Money
Connect With Your Community
Subscribe for stories that matter!
"*" indicates required fields