Yardley-Based Optinose’s Xhance Nasal Spray Wins FDA Nod for Chronic Rhinosinusitis
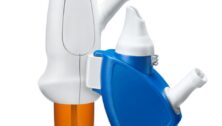
Yardley-based Optinose got the green light from the Food and Drug Administration for their flagship nasal spray Xhance, writes John George for the Philadelphia Business Journal.
Xhance already gained approval seven years ago to treat nasal polyps. The latest approval allows the product to be used to treat chronic rhinosinusitis.
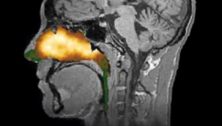
Xhance is officially the first FDA-approved medicine to treat the condition, which affects 30 million people in the United States.
Chronic Rhinosinusitis causes chronic inflammation in the paranasal sinuses and nasal cavity.
Before the FDA’s decision, Optinose’s shares had decreased slightly, less than 1 percent, standing at $1.88. However, in after-hours trading, the stock experienced a 7.5 percent increase, reaching $2.02.
Ramy Mahmoud, the CEO of Optinose, said that Xhance could transform the Yardley-based life sciences company into a profitable business specializing in ear, nose, throat, and allergy treatments.
“We believe Xhance has the potential to become part of the standard of care for the treatment of chronic sinusitis,” he said.
Read more about Yardley-based Optinose and their flagship drug Xhance’s approval in the Philadelphia Business Journal.
Dr. Ramy Mahmoud On a Novel Drug Delivery Method That Benefits Patients Prone to Sinus Infections
Connect With Your Community
Subscribe for stories that matter!
"*" indicates required fields