FDA Cites ‘Breakthrough’ Helius Medical Technologies Device for Helping Stroke and MS Patients Walk More Confidently

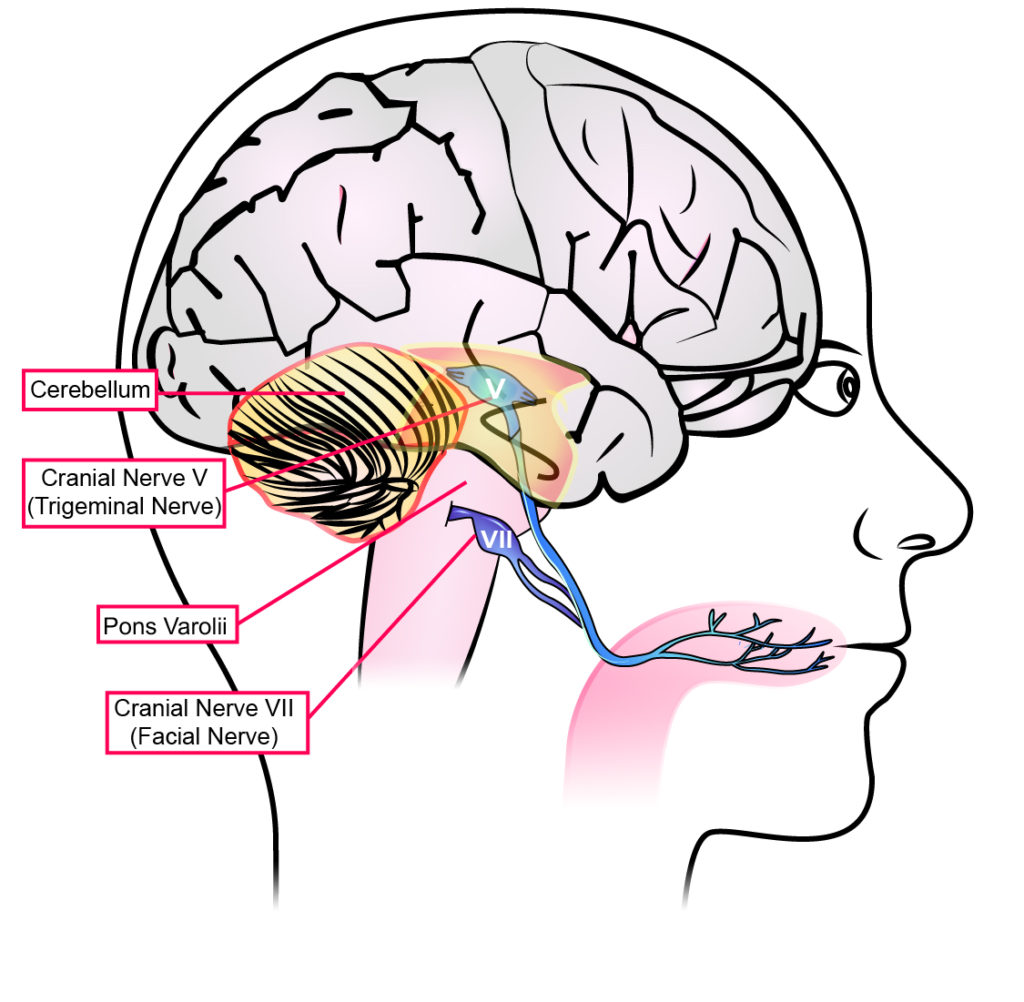
steady the entire central nervous
system, improving mobility.
Image via Helius Medical Technology.
The U.S. Food and Drug Administration (FDA) recognized a device produced by Newtown-based neurotech company Helius Medical Technologies, Inc. The solution, a portable neuromodulator stimulator (PoNS), enables patients with walking difficulties to steady their gait.
Its market includes patients who have had a stroke or have MS. Although not a sole answer to mobility issues, PoNS designers see it as a worthy adjunct to supervised exercise programs.
“We are very pleased to announce the receipt of Breakthrough Designation for our PoNS device to treat stroke-induced gait and balance deficits,” said Helius CEO, Dane Andreeff.
He continued: “Strokes are a large and growing cause of long-term disability in the United States. An estimated seven million Americans are living with stroke-related complications. And more than 80 percent of stroke survivors … develop gait impairment.”
The FDA’s recognition is an important milestone in Helius’ goal to provide a nondrug, nonimplantable treatment option for patients needing help performing daily tasks.
The FDA’s Breakthrough Devices Program offers manufacturers such as Helius an opportunity to interact with the administration’s experts. It provides manufacturers with feedback during treatment premarket review phases.
Connect With Your Community
Subscribe for stories that matter!
"*" indicates required fields