Pipersville Cardiac Patient Undergoes Clinical Trial of Heart Device

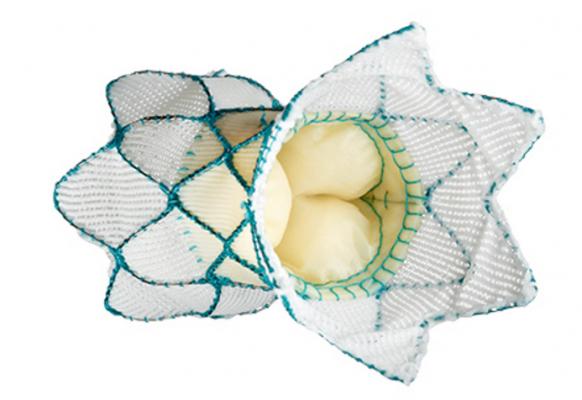
On March 21, U.S. Food and Drug Administration (FDA) gave thumbs up to a nonsurgical heart valve that ensures the proper direction of blood flow. This boon to babies born with congenital heart defects underwent clinical trial on patients that included a 20-year-old from Pipersville, reports Ali McPherson at WHYY.
Jack Hurley played sports and felt healthy. But he suffered from heart disease caused by a flaw that affected his cardiac performance from birth.
“I never noticed anything different between me or any of my friends that didn’t have heart disease,” said Hurley. “It was just another thing that I had to live with; it didn’t really impact my life at all.”
At three months old, Hurley underwent surgery to install a valve for the congenital heart defect that affecting his blood flow. Because that surgery was successful, he lived healthily into his teen years with no more operations.
Eventually, Hurley said, his medical team discussed replacing that original valve. But his family sought to postpone the procedure. They were eager to see their high-school athlete sidelined. Physicians okayed the pause.
By the time he was 18 and ready, a new technology had become available. Hurley agreed to take part in the clinical trial of a new technology called the Harmony Transcatheter Pulmonary Valve (TPV). Its introduction into the coronary system is nonsurgical, resulting in a faster healing time.
Hurley’s decision to undertake this new treatment has far-reaching implications. Expectations are that it can benefit roughly 40,000 babies each year who, according to the CDC, have congenital defects.
More information on this breakthrough advance is available at WHYY.
Connect With Your Community
Subscribe for stories that matter!
"*" indicates required fields